Directed Differentiation and Disease Modeling
Human pluripotent stem cell (hPSC)-based models hold tremendous potential for studying human development and disease. Directed differentiation and disease modeling are two key methods for using hPSCs in drug discovery, cell therapy validation, and disease research.
- Directed differentiation of hPSCs refers to the in vitro differentiation of these cells toward a specific cell type through defined cell culture conditions. Directed differentiation is achieved by the addition of specific growth factors or small molecules.
- Disease modeling is an approach to study diseases using cells that display relevant pathological features. Disease modeling using hPSCs can be achieved by reproduction of a disorder-associated mutation with gene editing, isolation of embryonic stem cells (ESCs) from affected blastocysts; or generation of induced pluripotent stem cells (iPSCs) from patients’ somatic cells.
Explore the resources below to support your disease modeling studies using hPSCs.
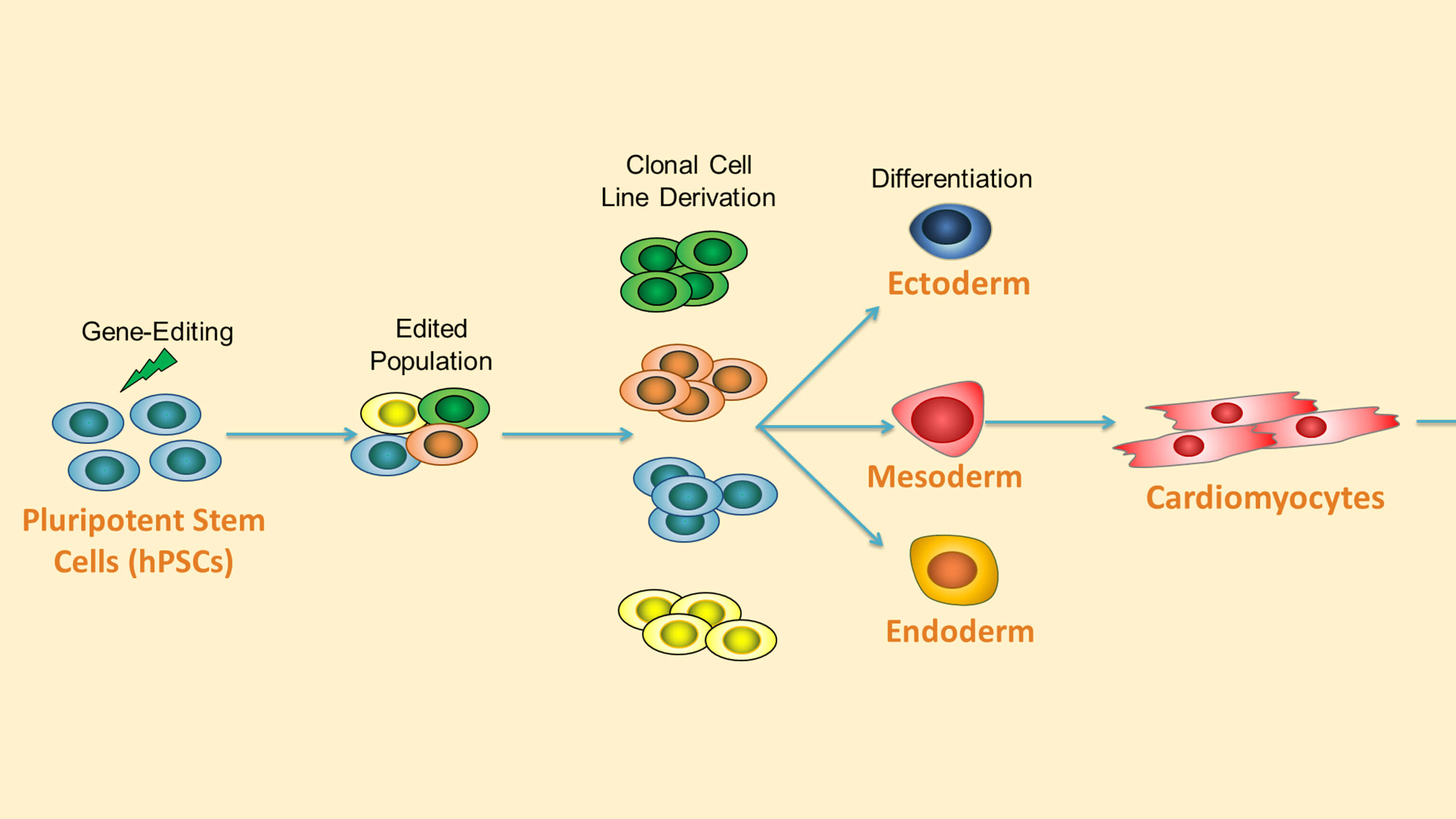
Highly Efficient Single-Cell Human Pluripotent Stem Cell Cloning and Robust Cardiomyocyte Differentiation
Recorded during the ISSCR 2017 Innovation Showcase in Boston, this tutorial highlights human pluripotent stem cell (hPSC) gene-editing and cardiac differentiation workflows using the CloneR‚Ñ¢ supplement and the STEMdiff‚Ñ¢ Cardiomyocyte System. This talk is presented ∫£Ω«∆∆Ω‚∞Ê‚Äô Dr. Adam Hirst, scientist for pluripotent stem cell biology, and Dr. Vincenzo Macri, senior scientist for cardiomyocyte stem cell biology.
View Now >- Genome Editing From Modeling Disease to Novel TherapeuticsThe use of genome editing tools to generate specific DNA variants in hPSCs provide more accurate modeling of human disease when differentiated into human-derived tissues. View this webinar presented by Dr. Chad Cowan at Harvard University, who discussed the design and use of TALENs or CRISPR/Cas9 systems with hPSCs and the applications of modified hPSCs, including disease modeling and gene therapy.
- STEMdiff‚Ñ¢ Kits for Robust and Efficient Differentiation of hPSCs to Multiple Cell TypesDr. Melanie Kardel, scientist at ∫£Ω«∆∆Ω‚∞Ê Technologies, describes the use of STEMdiff‚Ñ¢ kits to differentiate human pluripotent stem cells to all three germ layers. This video was filmed during the Innovation Showcase at ISSCR 2016 in San Francisco.